The technology owner had developed a patented standalone AC battery with a proprietary electrode design that has both the characteristics of anode and cathode. This enables the battery to generate AC power (square / pulsed wave form) from a single battery and a single switch. In a typical direct current (DC) to AC power conversion configuration used for brushless DC motors (BLDC) in drones and electric vehicles – multiple DC batteries, switches, complex battery management system and inverter circuit are needed to generate 3-phase AC to power a BLDC. The novel AC battery uses a simpler circuit design that minimises battery management system, converters and inverters.The use of the third electrode enables the voltage within the battery cells to be divided by half, e.g. while there is 4V between anode and cathode within the conventional Li-ion battery, the electrode can divide the voltage into 2V each, leading to safer operations and longer cycle life. The technology owner is looking at integrating the Cockcroft-Walton Multiplier (CWM), an established circuit that generates high DC voltage from an AC input as part of the AC battery system. The technology owner aims to boost the voltage, e.g. from 1.85V to 20V for industrial drones with an additional cost of USD200, while achieving 30% higher battery capacity with the AC battery and CWM combination.The technology owner had already developed several prototypes including a 100mA pouch cell. They are currently working on optimising the thickness of the electrode and preparing for a pilot test in industrial drones. The technology owner is seeking technical collaboration to scale up the AC battery prototype, develop integrated AC battery with CWM, conduct pilot test in drones, e-bikes, or e-wheelchair and eventually to license their technology to battery or battery parts manufacturers.
The proposed heat management technology focuses on high power applications (above 2C) that result in battery overheating, which can cause significant reduction in lifetime, performance and safety hazards.Thermal Management System (TMS) - During normal operation of batteries, the battery cells emit heat, which could cause the temperature of the battery pack to rise drastically. Without a TMS in place, heat would be trapped in the battery pack and could cause cell-degradation, leading to shortened lifetime, decreased performance and fire hazard. The proposed thermal management solution overcomes battery-overheating issue. The solution consists of liquid cooling and a proprietary material that could effectively prevent fire propagation, extend lifetime and increase performance of the battery. Working Mechanism of TMS - The TMS works by dissipating heat away from the battery cells. The proprietary thermal material is dielectric and can be poured directly into any battery pack. As the material flows into the pack, the material envelops the cells and serves as a protective layer between the cells. The material solidifies when it cools. During battery operation, the material absorbs heat emitted by the battery cells. Heat is then dissipated from the material via a liquid cooling circuit integrated in the TMS.The technology provider is actively seeking potential partnerships and technology licensing for its (i) proprietary TMS and (ii) standard battery module that consist of the TMS. The technology provider is also open to working with potential partners to fast-track their Second Generation phase change material (PCM) development.
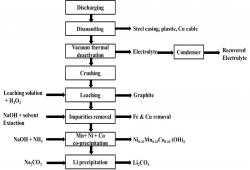
Lithium-ion batteries (LIBs) have been the preferred portable energy source in recent decades. The tremendous growth in the use of LIBs has resulted in a great number of spent LIBs. Disposal of these spent LIBs will cause serious environmental problems due to hazardous components such as heavy metals and electrolytes. Materials contained in the spent LIBs are valuable resources and could be recycled by proper technologies. Current methods are not suitable for LIB recycling due to slow process, low purity of the products (low profits) and the use of non-environmental friendly leaching reagents.The proposed LIB recycling technology is based on a co-precipitation process and control system which can process various types of spent LIBs including lithium cobalt oxide (LCO), lithium manganese oxide (LMO), lithium nickel manganese cobalt oxide (NMC) and lithium nickel cobalt aluminium oxide (NCA). The co-precipitation method allows the recovery of cathode metal salts in their original form, without separation of the metal elements. The obtained metal salts could then serve as the precursor for synthesis of new cathode material. In summary the process recovers the following products at more than 99% purity levels: (a) graphite and (b) cathode metal salts e.g. LiCo1/3Ni1/3Mn1/3O2, NiCO3, MnCO3, CoC2O4, and Li2CO3.The technology provider is seeking a partner who is willing to fund the prototype development and become an early adopter of the technology. Preferably, the partner should have access to spent LIB sources to support the trial.
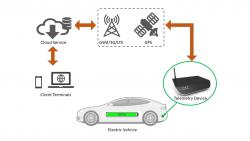
The smart telemetry is a system for remote monitoring of the status and parameters of the battery system in electric vehicles. The system front-end comprises of Global Positioning System (GPS) and 3rd Generation/4th Generation cellular communication (3G/4G) enabled devices with built-in sensors, to provide battery stack monitoring and data-logging capabilities. Data collected are relayed in real-time to data centre at the back-end. The system is a plug and play setup and comes with remote system recovery function from the server.The technology is connected to the car's battery management system to collect the basic battery data through CAN bus for State-of-Charge (SoC) and State-of-Health (SoH) determination. The algorithm developed and built in on-board to determine the SoC and SoH of the battery pack, can support individual cell level. The technology will monitor and diagnose different parameters (i.e. Voltage, Temperature, SoC, SoH) of the individual battery cell in real time, and provide notifications of the failure/defective cell.
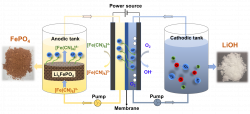
With the retirement of massive amount of end-of-life lithium ion batteries (LIBs), proper disposal of the hazardous wastes and cost-effective valorization of useful materials have become increasingly pressing and attracted extensive attention worldwide. The state-of-the-art recycling technologies, which are generally based on chemical leaching methods, have critical issues of enormous chemicals consumption, secondary pollution and tedious procedures.The technology relates to an innovative redox targeting-based process for the recycling of spent lithium iron phosphate (LiFePO4) batteries. With 0.20M of ferrocyanide [Fe(CN)6]3- solution as a selective and regenerative redox mediator, LiFePO4 is readily broken down into FePO4 and Li+ via the redox-targeting reaction. An Li-removal efficiency of 99.8% has been achieved with 50 minutes reaction at ambient conditions. The reacted redox species [Fe(CN)6]4- are instantaneously regenerated on the electrode for subsequent round of reaction while Li+ ion is separated from the counter electrode compartment as lithium hydroxide (LiOH). The technology provider is currently seeking industry partner to scale-up and commercialise the technology.