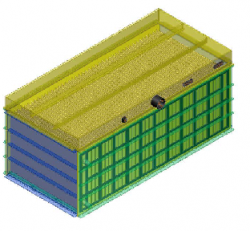
With the wide deployment of renewable energy harvesting devices, such as solar cells and wind turbines, there is an urgency to develop efficient and economical energy storage systems to stabilize the intermittent and often unpredictable primary power sources before the power can be channeled to the grid safely or utilized for on-site loads. Redox flow batteries (RFBs) are regarded as promising electrochemical energy storage devices due to their special features of separable energy and power capacity. However, redox flow batteries tend to have lower energy densities than integrated cell architectures. Here our inventions introduce the novel engineering design to and reduce bulk resistance with no significant increase in flow resistance, obtaining uniform flow throughout the battery cell and improving the overall system efficiency.
As the consumption of lithium-ion batteries (LIBs) for the transportation and consumer electronics sectors continues to grow, so does the pile of battery waste. Lithium-ion battery waste retains value particularly in the form of metal ions in the cathode part of the device, but few standardised methods exist to extract, recover, and reuse these precious metals. Such metals can be extracted using environmentally-friendly deep eutectic solvents which is safer than other corrosive hydrometallurgical methods which typically use strong acids, or high-energy pyrometallurgical methods which incinerate and grind waste battery material at temperatures beyond 1000˚C. The deep eutectic solvents (DES) can be made from commercially available commodities such as choline chloride and ethylene glycol which makes them good candidates for industrial scales. The battery recycling industries could benefit from the use of safer solvents that can still effectively extract and recover precious metals from spent lithium-ion batteries for reuse in other applications.Starting with disassembly of the LIB, cathode waste is inserted into a DES, which is then heated and stirred. Extraction of cobalt and lithiumm ions occurs through dissolution, and at this step, aluminium foil, binder and conductive carbon can be recovered separately when the leachate is filtered. Cobalt compounds can then be recovered either through precipitation or electrodeposition, allowing reutilization of these valuable materials.
Environmental pollution concerns and high fuel cost is driving the car industry towards Electric Vehicle (EV). Li-ion cell is a common adopted energy source for EV. However, Li-ion cells required proper temperature control to function properly. A key factor that affects the battery is temperature. < 0°C: difficult or impossible for charging >60°C: difficult for discharging and risk of degradation, shortened service life >70°C ~ 90°C: will trigger a self-heating reaction with internal cell faults with risk of thermal runaway, presenting safety hazards.Most Li-ion battery achieves their rated capacity at 20~25°C and their capacity will drop ~10% for every increase of 10°C. Regulating the battery temperature during continuous charge and discharge is a challenge, especially in temperate climates.Existing cooling solutions consist of the battery modules sitting on or attached to heat sinks that are in turn cooled by a coolant loop. The drawbacks are that the cooling efficiency is low, and the effectiveness is poor, since only a small part of each module receives the cooling effect. Besides, heat sinks are generally thick and heavy due to the coolant loop. The result is that temperatures will differ from module to module, cell to cell. Even within the same cell, different regions may have different temperatures.Battery packs used in EVs are constrained by space and weight, so cooling systems for the battery packs must be compact and lightweight, and yet meeting the cooling requirements.Our patent granted technology is able to carry coolant to each individual cell in a compact structure. This ensures consistency and uniformity of heat transfer from each cell in a battery pack, extending their lifespan and safety by allowing them to operate in their optimum temperature range (10 ~ 35°C), Charging and discharging can also take place in all ambient temperature.
Lithium ion batteries, which are the most widely used among the secondary battery types, have high capacities and cycling life. The theoretical capacity of lithium ion batteries is expected to remain at the same value in each cycle without changing the ingredients. However, this does not take place as expected and lithium ions in the battery can be produced or consumed by side reactions during charging/discharging. When the capacity stability of the battery deteriorates, there is a loss of capacity and a significant decrease in the performance of the battery occurs in long cycles. Anode and cathode materials of the batteries directly affect the capacity and cycle life. Therefore, it is very important to improve the anode and cathode materials in order to maintain stability and improve battery performance.The technology described herein is related to the development of high capacity lithium ion batteries based on silicon-based anode and lithium rich cathode materials. The anode is a silicon-based material that contains a conductive polymer additive. Polymer-Si is a porous material with a flexible shell structure, thus preventing the volumetric expansion of silicon. The cathode has been developed in special stoichiometry to maximize capacity, thermal stability and capacity retention rate. The technology provider is seeking industry partners to test-bed and commercialize the patent-pending technology.
Ships coming into docks are required to undergo hull inspections and dock position checks. This task is traditionally performed by human divers. Recently, remotely operated underwater vehicles (ROVs) have been deployed to aid in this process. However, conventional ROVs require the use of long umbilical cables or tethers to power the vehicles from shore or from floating buoys. This causes significant power losses.The proposed technology relates to an onboard smart underwater battery power system for ROVs. It removes the need for tethering for ROVs, which increases the energy efficiency and overall portability of the vehicles.The technology owner is seeking partners to collaborate in various modes including technology licensing, consultancy project, research collaboration, etc.